Tuesday, September 9, 2008
Chapter 5 Review
1. Which of the following is not an organic molecule?
c) water
2. Which of the following terms includes all the other terms on this list?
b)carbohydrate
3. Which term is the most appropriate to describe a molecule that dissolves easily in water?
c) hydrophilic
4. Cholesterol is an example of what kind of molecule?
b) lipid
5. The 20 amino acids vary only in their
b) side groups
6. A specific reactant an enzyme acts upon is called the
d) substrate
7. An enzyme does which of the following?
b) lowers the activation energy of a reaction
8. Besides satisfying your hunger, why else might you consume a big bowl of pasta the night before a race?
Besides satisfying your hunger, you may also consume a big bowl of pasta because your body wants energy for the next day.
9) How are glucose, sucrose, and starch related?Glucose and starch are both chains of many glucose monomers. Glucose, sucrose, and starch are all sugars.
10) What are steroids? Describe two functions they have in cells.
Steroids are lipid molecules in which the carbon skeleton forms four fused rings.
1. They circulate in your body as chemical signs.
2. The steroids in males and females causes the major differences in appearance between male and female mammals.
11)How are polypeptides related to proteins?
Polypeptides are cells that create proteins by linking amino acids together into a chain.
12)How does denaturation affect the ability of a protein to function?
Denaturation can cause a protein to unravel and lose its normal shape.
14)
a. One product of this reaction is represented by a question mark. Which molecule is it?
water
b. What is this kind of reaction called? Explain.
This kind of reaction is called a dehydration reaction. It is when water is removed from amino acids to bond them.
c. If an amino acid were added to this chain, at what two places could it attach?
The OH on the right side of the reaction or the H on the other part of the reaction.
15)
a) At which temperature does enzyme A perform best? Enzyme B?
Enzyme A performs best at 37 degrees celsius and Enzyme B performs best at around 78 degrees celsius.
b)Knowing that one of these enzymes is found in humans and the other in thermophilic (heat-loving) bacteria, hypothesize which enzyme came from which organism.
Enzyme A is thermophilic and Enzyme B is found in humans.
c)Propose a hypothesis that explains why the rate of the reaction catalyzed by enzyme A slows down at temperatures above 40 degrees celsius.
The reaction slowed down and ended and that was why the reaction catalyzed by enzyme A slows down at temperature above 40 degrees celsius.
Sunday, September 7, 2008
Summary of Chapter 5.5

Wednesday, September 3, 2008
Summary of Chapter 5.4
 Vocabulary:
Vocabulary:protein: a polymer constructed from a set of just 20 kinds of monomers called amino acids
amino acid: monomer that makes up proteins; contains carboxyl and amino functional group
polypeptide: chain of linked amino acids
denaturation: loss of normal shape of a protein due to heat or other facts
Proteins perform most functions in
 cells. They are responsible for most of the day-to-day functioning of organisms. They form hair and fur, make up muscles, and provide long term storages. Proteins defend the body from harmful microorganisms. Amino acids are monomers consiting of a central carbon atom bonded to four partners ( a carbon atom which forms four covalent bonds ). Cells create protein by creating a polypeptide. Each link is created by a dehydration reaction between the amino group of one amino acid and the carboxyl group of the next amino acid in the chain. Proteins can be formed by one more polypeptide chains. Proteins in their simple form of amino acids linked cannot function properly. Hydrophilic amino acids are towards the outside edges of protein while hydrophobic amino acids are towards the center of the protein. Any change in temperature, pH, or other qualities of environments can causea protein to unravel and lose its normal shape.
cells. They are responsible for most of the day-to-day functioning of organisms. They form hair and fur, make up muscles, and provide long term storages. Proteins defend the body from harmful microorganisms. Amino acids are monomers consiting of a central carbon atom bonded to four partners ( a carbon atom which forms four covalent bonds ). Cells create protein by creating a polypeptide. Each link is created by a dehydration reaction between the amino group of one amino acid and the carboxyl group of the next amino acid in the chain. Proteins can be formed by one more polypeptide chains. Proteins in their simple form of amino acids linked cannot function properly. Hydrophilic amino acids are towards the outside edges of protein while hydrophobic amino acids are towards the center of the protein. Any change in temperature, pH, or other qualities of environments can causea protein to unravel and lose its normal shape.Concept Check 5.4
1. Give at least two examples of proteins you can "see" in the world around you. What are their functions?
Two examples are animal hair and fur, and the muscles. The animal hair and fur protects your outside part ( the skin ) and the muscles strengthen you.
2. Relate amino acids, polypeptides, and proteins.

Protein is a polymer constructed from set of 20 kinds of monomers called amino acids, and polypeptides are chains of linking amino acids, which makes proteins.
3. Explain how heat can destroy a protein. Heat can destroy proteins when the polypeptide chains become tangled with one another. Heating unfolds proteins because most of the forces that maintain folding are weak attractions between pairs of side groups, and between side groups and water. Hot molecules collide with force to overcome the weak attraction. Because the function of proteins depend on its shape, they lose their ability to work properly.
4. Which parts of an amino acid's structure are the same in all amino acids? Which part is unique? All amino acids have the hydrogen atom, an amino, and the carboxyl group. The fourth part is unique because it creates the protein's properties.
Summary of Chapter 5.3
 Vocabulary:
Vocabulary:lipid: one of a class of water-avoiding compounds
hydrophobic: water fearing
fat: organic compound consisting of a three-carbon back (glycerol) attached to three fatty acids
saturated fat: fat in which all three fatty acids chains contain the maximum possible number of hydrogen atoms
unsaturated fat: fat with less than the maximum number of hydrogens in one or more of its fatty acid chains
steroid: lipid molecules with four fused carbon rings
cholesterol: steroid molecule present in the plasma membranes of animal cells

Lipids include fats and steroids. Lipids are hydrophobic. Fats are stored for later use and the fatty tissues cushion your organs. All the carbon atoms in the fatty acid chains form single bonds with each other, and the rset of their bonds are with hydrogen atoms.
animal fats: lard and butter (both are saturated)
unsaturated fats: fruits, vegetables, fish, corn oil, olive oil, and other vegetable oils
Steroids circulate in your body as chemical signals. Cholesterol is the best-known steroid. Cholesterol has a bad reputation because high levels of cholesterol can increase risks of heart and blood vessel diseases.
Concept Checks:
1. What property do lipids share? Lipids share the property of being hydrophobic ( meaning water-avoiding ).

2. What are the parts of a fat molecule? A fat molecule consists of a three-carbon backbone called glycerol attached to three fatty acids that contain long hydrocarbon chains.
3. Describe two ways that steroids differ from fats. In the body, fats store energy for later use as fatty tissues cushion your organs and steroids circulate around your body as chemical signals. Fats are of glycerol attached to three fatty acids while a steroid is a lipid where the carbon skeleton forms four fused rings.
4. What does the term unsaturated fat on a food label mean? Unsaturated fat means that it contains less than the maximum number of hydrogen atoms in one or more of its fatty acid chains because some of the carbon atoms are double bonded to each other.
Tuesday, September 2, 2008
Summary of Chapter 5.2
 Vocabulary:
Vocabulary:
carbohydrate: an organic compound made of sugar molecules
monosaccharide: simple sugars that contain just one sugar unit
disaccharide: sugar with two monosaccharides
polysaccharide: long polymer chain made up of simple sugar monomers
starch: a polysaccharide found in plant cells that consists entirely of glucose monomers
glycogen: excess sugar in a form of a polysaccharide
cellulose: polysaccharide consisting of glucose monomers that reinforce plant-cell walls
This section discusses about the definition of carbohydrates and how carbohydrates provide energy. In a dehydration reaction, cells create two monomers, becoming disaccharides ( the main type of disaccharide is sucrose ). Long polymer chains of simple sugar monomers are polysaccharides, such as starch, a polysaccharide found in plant cells consisting entirely of glucose monomers. When plants break down s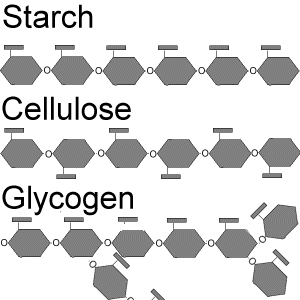 tarch molecules, the glucose becomes available to use. Animals and humans store sugar in a type of polysaccharide called glycogen. Just like starch, glycogen is also a chain of many glucose monomers. When the glycogen is needed in animals, the body breaks down the energy from glycogen granules releasing glucose. Cellulose are polysaccharides that protect cells and stiffens plants. Cellulose is also made of glucose monomers. Most animals and people cannot digest cellulose because they do not contain the molecule necessary to break the bonds of the glucose monomers in glucose. All carbohydrates are hydrophilic. Starch comes from plants and glycogen comes from humans and animals.
tarch molecules, the glucose becomes available to use. Animals and humans store sugar in a type of polysaccharide called glycogen. Just like starch, glycogen is also a chain of many glucose monomers. When the glycogen is needed in animals, the body breaks down the energy from glycogen granules releasing glucose. Cellulose are polysaccharides that protect cells and stiffens plants. Cellulose is also made of glucose monomers. Most animals and people cannot digest cellulose because they do not contain the molecule necessary to break the bonds of the glucose monomers in glucose. All carbohydrates are hydrophilic. Starch comes from plants and glycogen comes from humans and animals.
Concept Checks
1. Explain the difference between a monosaccharide and a disaccharide. Give an example of each. Monosaccharides contain one sugar unit ( simple sugars ) and a disaccharide contains two sugar units ( double sugars ). An example of a monosaccharide is fructose and an example of a disaccharide is sucrose.
2. Compare and contrast starch, glycogen, and cellulose. Starch, glycogen, and cellulose are all polysaccharides. Glycogen is found in animals and humans and a glycogen polymer is more highly branched than starch polymer. Cellulose is found in plants, just like starch, but they work as building materials. Starch, glycogen, and cellulose are all made of glucose monomers.
3. How do animals store excess glucose molecules? Animals store excess glucose molecules in glycogen. When the body needs energy, it breaks down glycogen granules, releasin glucose.
Summary of Chapter 5.1
organic molecule: carbon based molecule

inorganic molecule: non carbon based molecule
hydrocarbon: organic molecules that are composed of only carbon and hydrogen
functional group: a group of atoms within a molecule that interacts in predictable ways with other molecules
hydrophilic: attracting water molecules
monomer: small molecular unit that is the building block of a larger molecule
polymer: long chain of small molecular units (monomers)

Carbon is the main ingredient of organic molecules. They may form bonds with other carbon atoms to make an endless variety of carbon skeletons. Organic molecules are important, such as hydrocarbon. Hydroxyl groups are hydrophilic so they often are surrounded by water molecules in an aqueous environment. Large molecules are built by monomers. Cells connect monomers into polymers and every living cell has thousands of polymers. When a monomer is added to a polymer, a water molecule releases, resulting into a dehydration reaction. . Polymers can also be broken down to receive their energy. Cells break bonds between monomers by adding water to them. A hydrolysis reaction is when water is used to break down the polymer.  Water is removed to build a polymer, and water is added to break it down.
Water is removed to build a polymer, and water is added to break it down.
Concept Check
1. Draw a molecule that has a three-carbon skeleton and a hydroxyl group on the middle carbon. (Hint: The molecule's formula is C3H80)
2. Explain the connections between monomers and polymers. Monomers are small molecular units that link together into long chains called polymers.
3. What molecule is released during the construction of a polymer? What is this reaction called? Each time a monomer is added to a chain, a water molecule is released. This reaction is called a dehydration reaction.
4. Draw at least three ways in which five carbon atoms could be joined to make different carbon skeletons.




